AG Prof. Dr. Ralf Seemann
| |
|
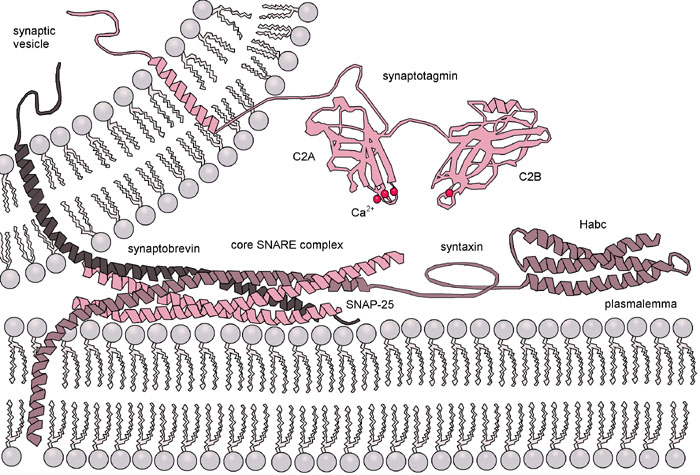
The role of specific SNARE amino acids in the transmembrane domain (TMD) for the protein complexation and for vesicle fusion kinetics shall be clarified in this project.
Our colleagues Dieter Bruns and Yvonne Schwarz (Molecular Neurophysiology) found that v-SNAREs present natural mutations, which depend on vesicle size ranging from 50 nm to granules as large as ~800 nm; interestingly these natural variations in TMD sequence influence the mechanical properties of SNAREs. Based on their results, Dieter Bruns and Yvonne Schwarz speculated that the role of such TMD variants is to compensate bilayer curvatures during fusion pore formation, which is varying as function of vesicle size.
We aim to test this hypothesis, by studying the nature of the nascent fusion pores and how they influence fusion kinetics. Two distinct hypotheses have been proposed to describe the nature of nascent fusion pores: the lipid-stalk fusion hypothesis where a purely lipid pore is formed, and the proteinaceous fusion-pore model when a fusion pore is lined by the transmembrane domains of SNARE proteins. The lipid-stalk hypothesis is support by molecular dynamic simulation, and the fact that membrane fusion can occur without proteins. The second model is supported by the fact that TMD SNARE mutations can affect content released in a predictable manner. The debate is still vigorous between these two models, as transient fusion pores are difficult to observe due to their size and their short lifetime. Using our microfluidic approach, we can determine the nature of the fusion pore depending on the specific TMD mutation.
Group members on this project: Navid Khangoli, Dr. Jean-Baptiste Fleury, Prof. Dr. Ralf Seemann
Internal collaborations: Dieter Bruns and Yvonne Schwarz (Molecular Neurophysiology)
Funding: DFG-CRC 1027