AG Prof. Dr. Ralf Seemann
| |
|
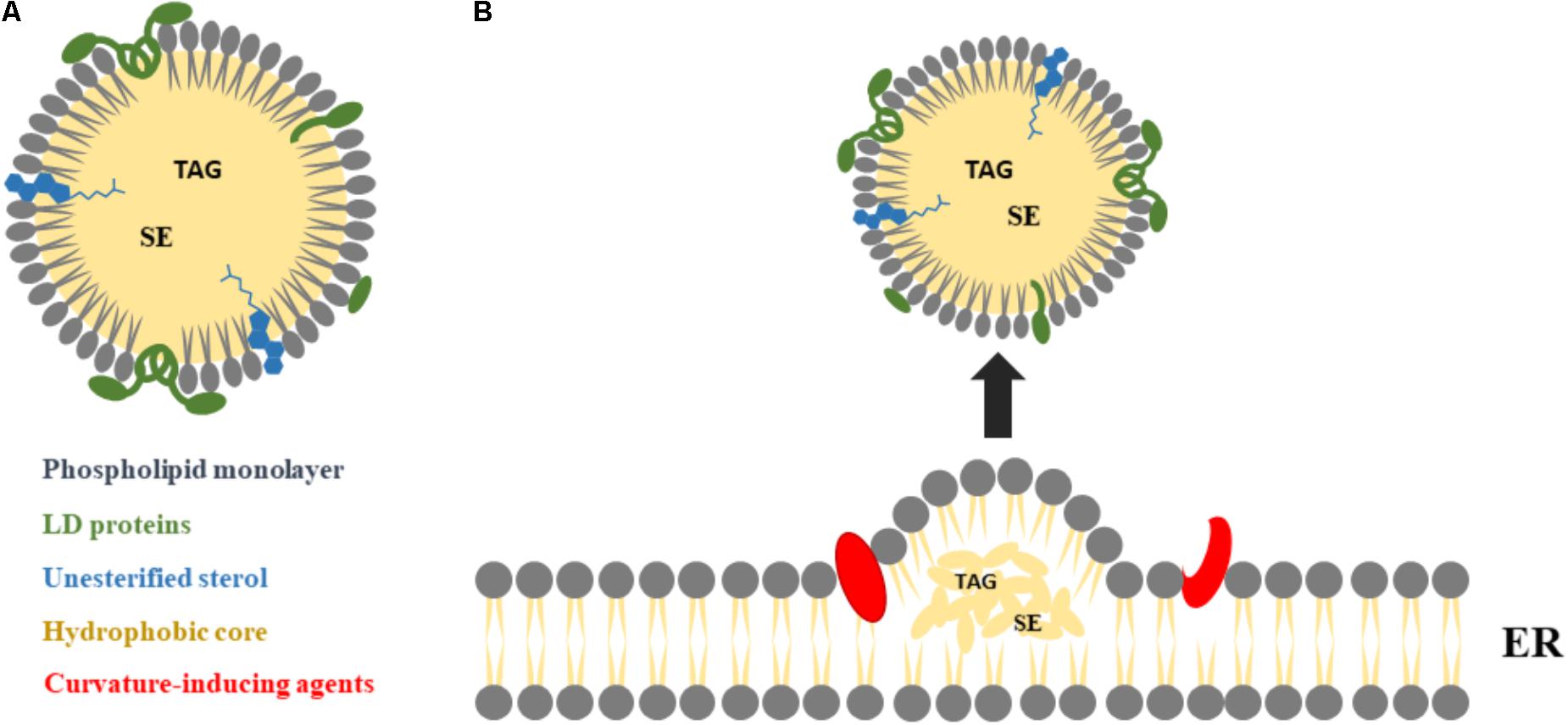
Within the CRC 1027 and in collaboration with our colleagues Bianca Schrul (Center for Molecular Signaling), Jochen Hub (Theoretical Physics) and Rober Ernst (Center for Molecular Signaling), we aim at employing a microfluidic scheme to reconstitute a type of artificial sub-organelles: the lipid droplet (LD). LDs are cytoplasmic organelles specialized for the storage of metabolic energy in the form of neutral lipids such as triglycerides and sterol esters. LDs biological functions are performed by proteins that are dynamically moving between the LD surface and the bilayer, they are sitting in. In cells, the motion of these proteins seems to be controlled by the metabolic need of cells. However, the conditions and mechanisms underlying the partitioning are not understood. We aim at understanding the dynamic organization of different classes of proteins between bilayer and LD covering monolayer and unravel underlying (universal) conditions controlling the partitioning by varying biophysical parameter like composition of phospholipid bilayer (including curvature inducing phospholipids), composition of the LD, density of proteins (to test crowding effects), lipid surface tension, bilayer tension, symmetric and asymmetric bilayer.
Group members on this project: Sevde Puza, Shima Asfia, Dr. Jean-Baptiste Fleury, Prof. Dr. Ralf Seemann
External collaborations: Bianca Schrul (Center for Molecular Signaling), Jochen Hub (Theoretical Physics) and Rober Ernst (Center for Molecular Signaling),
Funding: DFG-CRC 1027